Advantage India
Skilled human
capital
*With a total population of 1.4 billion, 47% being under the age of 25, India has a large pool of young and skilled workforce.
*India has a large reservoir of scientific human resources including scientists and engineers.
*Biotechnology has emerged as a trending career option among the youth. According to a survey of Class 12th students in Delhi, Biotechnology was ranked as the preferred stream at No.4/5.
Infrastructure
facilities
* India currently has 12 DBT-supported biotechnology parks and 95 BIRAC-supported bio-incubators (75 Bio-NEST and 20 E-Yuva centres) as of 2025.
* Union Minister of Earth Sciences Dr. Jitendra Singh inaugurates the Northeast's first International Biotech Conclave.
* Department for Promotion of Industry and Internal Trade (DPIIT) signed an MoU with Thermo Fisher Scientific to support over 500 biotech startups in the next three years through advisory, technology access, mentorship, and investor connect.
Policy
Support
* The National Biopharma Mission (NBM–i3), led by DBT and implemented by BIRAC with World Bank support, has a Rs. 2,137 crore (US$ 250 million) budget, supports 101 projects with 150+ organisations and 30 MSMEs, set up 11 shared facilities, and created 1,000+ jobs including 304 scientists, alongside the Genome India Programme sequencing 10,000 genomes.
* The government envisions creating biofoundries, Bio AI centres, and thematic hubs focused on bio-based chemicals, smart proteins, and carbon capture as part of policy support to foster biomanufacturing and innovation.
* The Cabinet has approved the merger of two DBT schemes into ‘Biotechnology Research Innovation and Entrepreneurship Development (Bio-RIDE)’, with a new Biomanufacturing and Biofoundry component, allocating Rs. 9,197 crore (US$ 1.1 billion) for 2021-22 to 2025-26 under the 15th Finance Commission.
Epidemiological
factors
* Patient pool expected to increase over 20% in the next 10 years, mainly due to rise in population.
* New diseases & lifestyle changes to boost demand for drugs and devices.
BIOTECHNOLOGY CLUSTERS
- Haryana
- Bengaluru
- West Bengal
- Maharashtra

Industry Contacts
- Department of Biotechnology, Ministry of Science & Technology
- Department of Science and Technology, Ministry of Science and Technology
- Biotechnology Industry Research Assistance Council (BIRAC)
- Council of Scientific and Industrial Research (CSIR)
- Association of Biotechnology Led Enterprises (ABLE)
- The Biotech Research Society, India
Posters
MORE
CATALYSING GROWTH
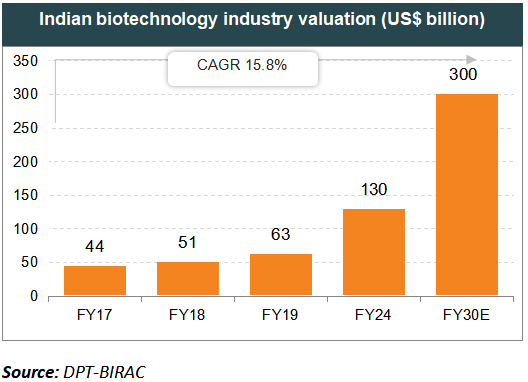
The Indian biotechnology industry amounted to US$ 63 billion in 2019 and is forecast to reach US$ 150 billion by 2025.
IBEF Campaigns
MORE
Aatmanirbhar Bharat Utsav 2024
Union Minister of External Affairs, Dr. S. Jaishankar and Union Commerce an...
Case Studies
MOREIBEF BLOG
MOREHow Green Hydrogen Will Shape Renewable Energy in India
Green hydrogen, a superior and a more sustainable alternative to fossil fue...
Cooperatives Rising: How Local Communities Are Shaping India’s Growth
The co-operative movement in India is a strong driving force of inclusive d...
India’s Aspirational Districts: Stories of Progress and People-Led Change
India’s Aspirational Districts Programme (ADP) was launched in Januar...