Advantage India
Cost
Efficiency
* India has emerged as the medial tourism hub of the world providing cost-effective treatments with the latest technology enabled by several pathbreaking reforms and provisions in healthcare sector.
* Access to affordable HIV treatment from India is one of the greatest success stories in medicine. India is one of the biggest suppliers of low-cost vaccines in the world, thereby rightly making it the ‘Pharmacy of the World’.
Economic
Drivers
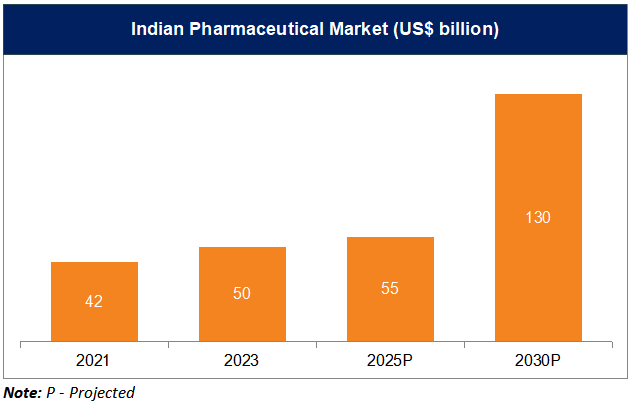
*According to Bain & Co, the Indian Pharmaceutical market stood at Rs. 4,71,295 crore (US$ 55 billion) in 2025 and is expected to grow to Rs. 10,28,280-11,13,970 crore (US$ 120-130 billion) by 2030.
*In FY24 domestic consumption was valued at Rs. 2,01,372 crore (US$ 23.5 billion).
*The pharmaceutical industry’s total turnover stood at Rs. 2,25,000 crore (US$ 26.26 billion) in FY25 lead by cardiac, gastrointestinal and anti-diabetic segments.
*India has the largest number of USFDA-compliant plants outside the US and over 2,000 WHO-GMP approved facilities, exporting to 150+ countries.
*CRDMO industry expected to double to Rs. 1,21,282 crore (US$ 14 billion) by 2028, reinforcing India’s role in global supply chains.
Policy
Support
*PLI scheme: Rs. 15,000 crore (US$ 2.04 billion) outlay (2020-21 to 2028-29) to boost manufacturing, investment, and product diversification.
*Strengthening of Pharmaceutical Industry (SPI): Rs. 500 crore (US$ 60.6 million) to support pharma clusters and MSMEs in productivity, quality, and sustainability.
*Under the Pradhan Mantri Bhartiya Janaushadhi Pariyojana, 16,912 Jan Aushadhi Kendras are operational as of June 30, 2025, with a target of 25,000 by March 2027, offering 2,110 medicines and 315 devices/consumables to promote affordable quality generic healthcare.
*Government disbursed Rs. 604 crore (US$ 69.76 million) under the PLI scheme in H1 FY25.
Increasing
Investment
*FDI policy: Up to 100% FDI allowed via automatic route for Greenfield pharma projects; up to 74% for Brownfield via automatic, beyond that with government approval.
*The Drugs & Pharmaceuticals sector received FDI inflow of Rs. 2,10,940 crore (US$ 24.62 billion) from April 2000-June 2025.
*Union Budget 2025-26: Allocation of Rs. 5,268 crore (US$ 602 million) for the Department of Pharmaceuticals, up 28.8% over previous budget estimates.
Major states for Pharmaceuticals
- Karnataka
- Maharashtra
- Gujarat
- Uttar Pradesh
- Delhi NCR
- Tamil Nadu
- Telangana

IBEF Campaigns
MORE
Aatmanirbhar Bharat Utsav 2024
Union Minister of External Affairs, Dr. S. Jaishankar and Union Commerce an...
Case Studies
MOREIBEF BLOG
MORERural Tourism in India: Empowering Villages and Reviving Local Economies
Rural tourism is emerging as a key driver of India’s inclusive growth...
Smart Textiles & Wearables: Weaving Tech into Tradition
The textile industry is dynamically changing. It is no longer a simple nece...
India's Developer Boom: Building the Future with AI and Innovation
India's developer community has reached an inflection point. With 17 mi...