Advantage India
Cost
Efficiency
* India has emerged as the medial tourism hub of the world providing cost-effective treatments with the latest technology enabled by several pathbreaking reforms and provisions in healthcare sector.
* Access to affordable HIV treatment from India is one of the greatest success stories in medicine. India is one of the biggest suppliers of low-cost vaccines in the world, thereby rightly making it the ‘Pharmacy of the World’.
Economic
Drivers
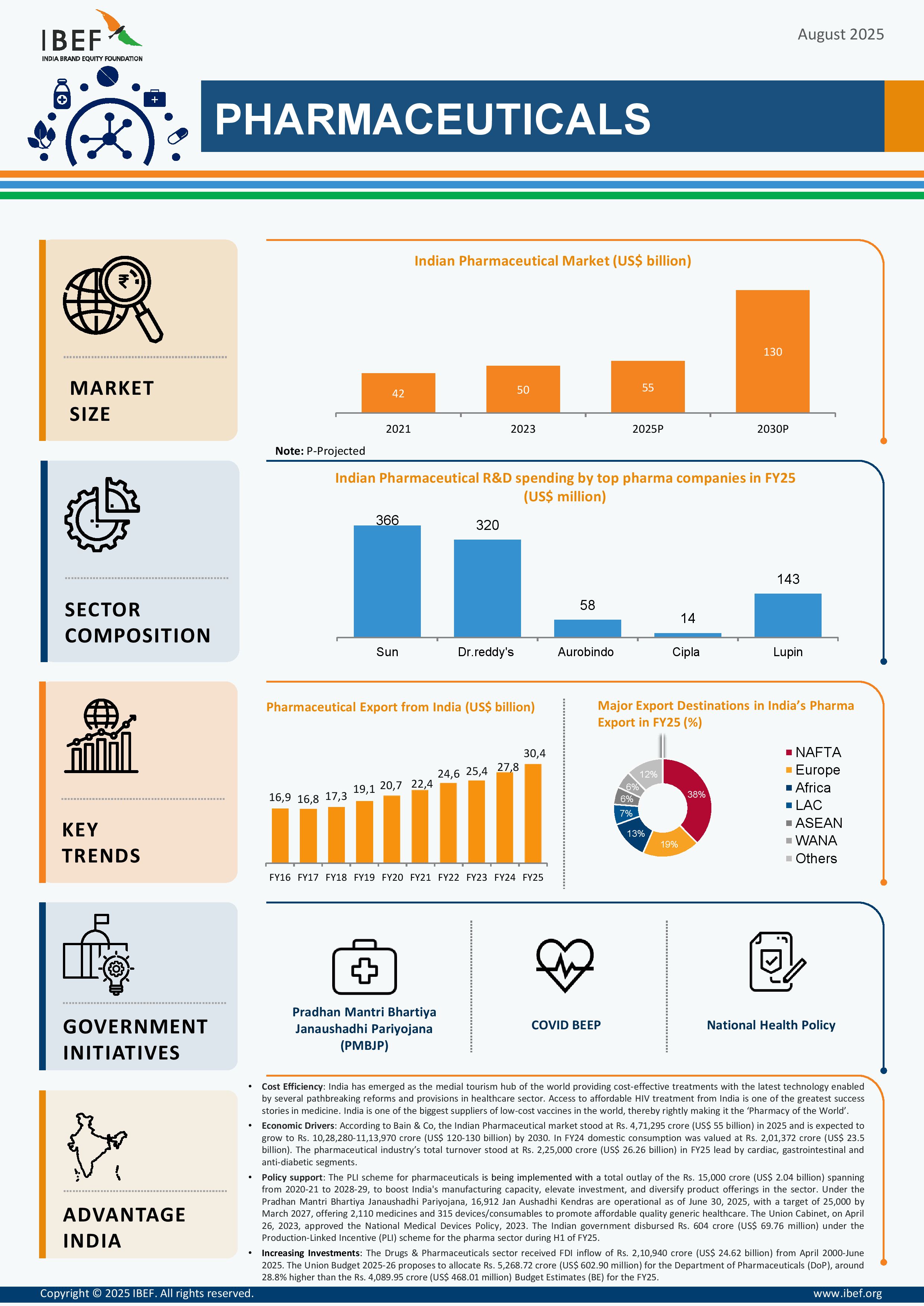
*According to Bain & Co, the Indian Pharmaceutical market stood at Rs. 4,71,295 crore (US$ 55 billion) in 2025 and is expected to grow to Rs. 10,28,280-11,13,970 crore (US$ 120-130 billion) by 2030.
*In FY24 domestic consumption was valued at Rs. 2,01,372 crore (US$ 23.5 billion).
*The pharmaceutical industry’s total turnover stood at Rs. 2,25,000 crore (US$ 26.26 billion) in FY25 lead by cardiac, gastrointestinal and anti-diabetic segments.
*India has the largest number of USFDA-compliant plants outside the US and over 2,000 WHO-GMP approved facilities, exporting to 150+ countries.
*CRDMO industry expected to double to Rs. 1,21,282 crore (US$ 14 billion) by 2028, reinforcing India’s role in global supply chains.
Policy
Support
*PLI scheme: Rs. 15,000 crore (US$ 2.04 billion) outlay (2020-21 to 2028-29) to boost manufacturing, investment, and product diversification.
*Strengthening of Pharmaceutical Industry (SPI): Rs. 500 crore (US$ 60.6 million) to support pharma clusters and MSMEs in productivity, quality, and sustainability.
*Under the Pradhan Mantri Bhartiya Janaushadhi Pariyojana, 16,912 Jan Aushadhi Kendras are operational as of June 30, 2025, with a target of 25,000 by March 2027, offering 2,110 medicines and 315 devices/consumables to promote affordable quality generic healthcare.
*Government disbursed Rs. 604 crore (US$ 69.76 million) under the PLI scheme in H1 FY25.
Increasing
Investment
*FDI policy: Up to 100% FDI allowed via automatic route for Greenfield pharma projects; up to 74% for Brownfield via automatic, beyond that with government approval.
*The Drugs & Pharmaceuticals sector received FDI inflow of Rs. 2,10,940 crore (US$ 24.62 billion) from April 2000-June 2025.
*Union Budget 2025-26: Allocation of Rs. 5,268 crore (US$ 602 million) for the Department of Pharmaceuticals, up 28.8% over previous budget estimates.
Major states for Pharmaceuticals
- Karnataka
- Maharashtra
- Gujarat
- Uttar Pradesh
- Delhi NCR
- Tamil Nadu
- Telangana

Posters
MORE
Pharmaceutical Companies in India
Indian pharma company have won around 40 per cent of all Abbreviated New Drug Approval (ANDA) from the US Food and Drug Administration (FDA) between january and july 2013.
IBEF Campaigns
MORE
Aatmanirbhar Bharat Utsav 2024
Union Minister of External Affairs, Dr. S. Jaishankar and Union Commerce an...
Case Studies
MOREIBEF BLOG
MORECooperatives Rising: How Local Communities Are Shaping India’s Growth
The co-operative movement in India is a strong driving force of inclusive d...
India’s Aspirational Districts: Stories of Progress and People-Led Change
India’s Aspirational Districts Programme (ADP) was launched in Januar...
Government Initiatives Boosting the Healthcare Industry in India
India has been undertaking sweeping reforms to strengthen its healthcare ec...