Quality Standard Certifications in Export: Sealing Trust with India’s Quality Certifications
India’s certification ecosystem is extensive, spanning statutory bodies, standardisation agencies, and accreditation councils. Meeting well-known global standards and certifications is often a requirement for entering foreign markets. Consistency in quality underpins buyer trust, and India’s certification regimes ensure products are consistently made to standard, reducing variability. For example, exporters who use inputs certified by Bureau of Indian Standards (BIS) and undergo Export Inspection Council (EIC) inspections can demonstrate “tested and verified” performance to overseas customers. Meeting these benchmarks helps exporters build credibility in global markets.
The Government of India has ensured that it promotes exports by facilitating easier certifications and aiding exporters throughout. Right from simplifying procedures to providing dedicated help, these efforts aim to strengthen businesses stepping out into international markets. Through this article, we aim to guide first-time exporters and enable them to create brand recognition abroad. For this to be practical and doable, besides written inputs from our side, we have also interviewed industry experts as well as successful exporters so that readers get through real-world insights.
India’s certification framework
India’s certification ecosystem spans statutory bodies, standardisation agencies, and accreditation councils. The BIS is the National Standards Body of India, formulating Indian standards and managing product certification schemes.Its hallmark (the Indian Standards Institution (ISI) standard mark) indicates conformity to relevant safety and performance criteria.
The EIC, under the Ministry of Commerce, oversees export quality control, notifies commodities subject to pre-shipment inspection, and certifies their compliance.
The Quality Council of India (QCI) runs accreditation boards for testing labs and certification bodies and also manages specialist schemes such as the India Conformity Assessment Scheme (i-CAS) for Halal exports, which was mandated for meat products in 2024. Together, these institutions set and enforce quality norms for Indian exports.


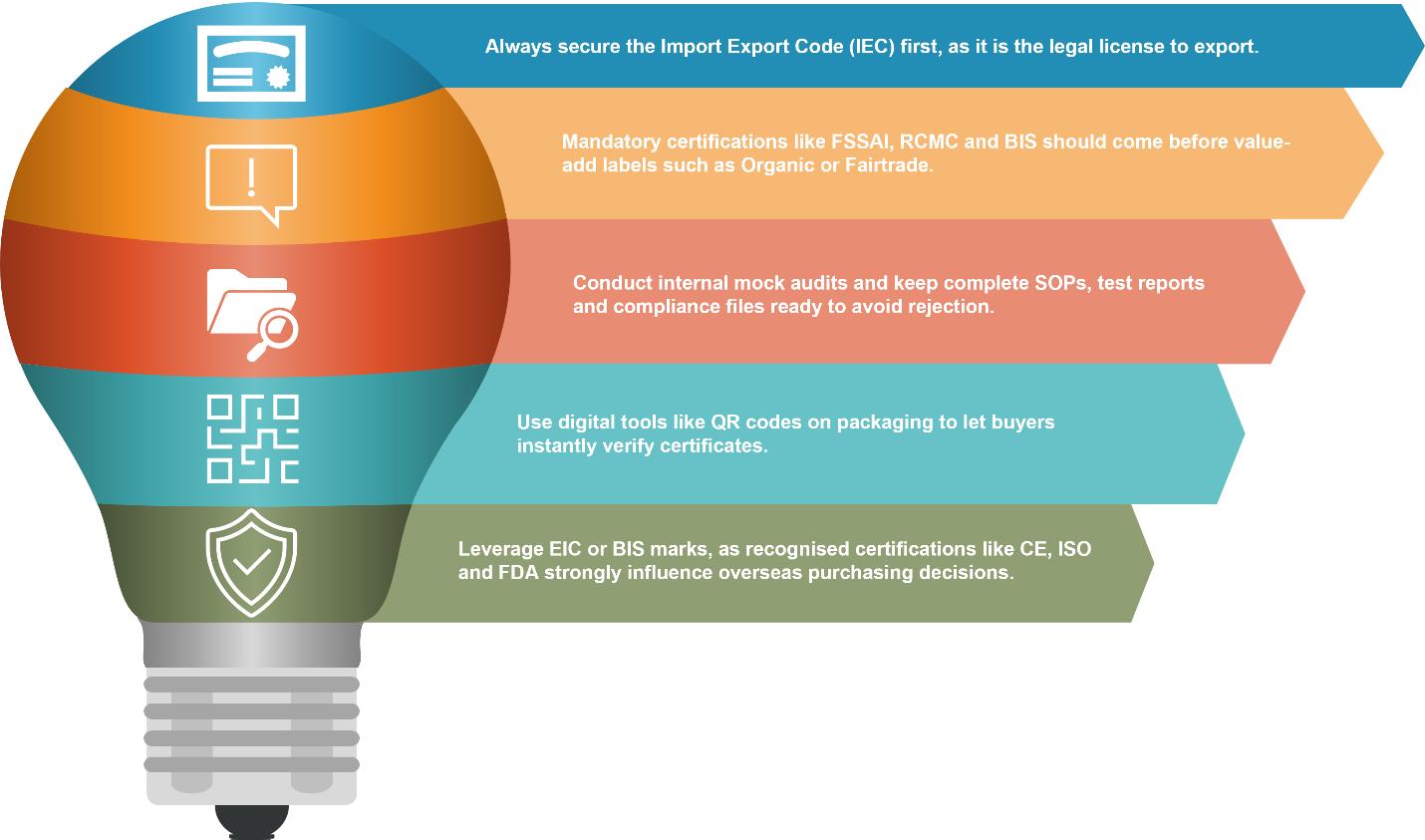
For first-time exporters, navigating this system can seem overwhelming. As Mr. Sidharth Agrawal, Chairman & Managing Director of Systematic Industries, explained, “The first and most important passport is the Import Export Code (IEC), the legal license to export.
After IEC, key certifications like ISO 9001 (quality), CE marking (safety) and BIS certification help products meet global standards. These certifications build buyer trust by showing your products are safe, reliable, and meet international rules.”
Adding to this, Mr. Gautam Raghuraman, Co-founder and Head of Sales (Domestic and Exports) of Beyond Snack, observed that certifications in export can be viewed in two categories: mandatory/legal and quality/safety.
“To export to any country we must have IEC, FSSAI, RCMC, PAN and GST. Market and product specific certificates are critical for demonstrating compliance and are often demanded by international buyers. Based on the target country, there will also be specific country-required certificates as well, such as Certificate of Origin, APEDA, Phytosanitary and Fumigation certificate and Health certificates.”
In the food and agriculture sector, additional certifications are particularly important. Mr. Sailesh Shah, Export Manager at Everest Food Products, pointed out that “In food and agri exports, FSSC 22000/BRC builds confidence in safety standards, while FSSAI provides the domestic baseline.”
EIC
EIC, established under the Export (Quality Control and Inspection) Act, 1963, plays a crucial role in ensuring that export products meet specified standards. It authorises two main types of certifications: consignment-wise inspections, where each shipment is sampled and tested, and Food Safety Management System (FSMS) certification, which is designed for exporters of food items.
In practice, exporters receive an EIC “Certificate for Export” when consignments pass tests, or they may obtain periodic FSMS certification for compliant factories. The EIC also issues Certificates of Origin under India’s preferential tariff schemes. As the apex quality body, the council advises the government on standards and inspection procedures and oversees a network of Export Inspection Agencies across Chennai, Delhi, Kochi, Kolkata, and Mumbai.
Recent reforms have significantly improved the exporter experience. Mr. Sailesh Shah explained that “Recent reforms under the EIC system have transformed the exporter’s experience. A new integrated digital portal offers e-certificates, traceability, and lab management. Regional labs in Ahmedabad (Gujarat), Faridabad (Haryana), Mangaluru (Karnataka) and Kakinada (Andhra Pradesh) are expanding capacity, cutting turnaround times. Blockchain pilots now enable overseas buyers to instantly verify Indian certificates, while low-risk categories are benefiting from self-certification schemes.”
BIS
BIS is India’s national standards body, responsible for harmonising standardisation, marking and product quality certifications. Through BIS, India develops Indian Standards (IS) for thousands of products, with the BIS product certification scheme providing the familiar ISI mark.
Manufacturers, including foreign firms, can obtain a BIS licence confirming that their goods meet the relevant IS. The presence of the BIS Standard Mark on a product acts as a quality-assurance signal, assisting exporters in assuring buyers of uniform standards. While many BIS schemes are voluntary, the government mandates certification for goods where safety, security or consumer protection is at stake. Having a BIS licence can boost credibility among exporters, particularly in markets where Indian standards are recognised or where alignment with global benchmarks is required.

BIS also plays a strong role globally. It is a founder member of the International Organization of Standardization (ISO), participates in the International Electrotechnical Commission (IEC), and serves as the national enquiry point of the World Trade Organization’s (WTO) Technical Barriers to Trade (TBT) Agreement.
This enables transparency and consultation about technical regulations influencing trade, ensuring Indian standards remain internationally aligned.
However, the certification process can be challenging for newcomers. As Mr. Sailesh Shah observed, “The road to certification, however, can be tricky. New exporters make mistakes by applying for certifications not relevant to destinations they target, submitting incomplete files or underestimating pre-audit intensity. To avoid costly rejection or delay, exporters will initially need to identify destination-country requirements, conduct internal mock audits, and have one-window file handy regarding SOPs, test reports, and compliance certificates.”
Avoiding delays or rejections requires preparation. Mr. Sidharth Agrawal explained, “To avoid issues: understand certification requirements fully, prepare accurate and complete documents, align products and processes with global standards, train the team on compliance, work with experienced consultants and conduct internal mock audits before official inspection.”
The value of recognised marks in international markets cannot be stated enough. According to Mr. Sidharth Agrawal, “Internationally recognised certifications such as CE, ISO and FDA significantly influence purchasing decisions, especially in countries with high regulations, such as the EU, North America, Middle East and Asia-Pacific.”

Key international benchmarks
The Indian exporters are usually required to comply with internationally recognised standards and certifications to enter the market. As an example of management practices, one can note ISO standards such as ISO 9001 on quality or ISO 22000/HACCP on food safety. Electronics and machinery might need CE marking (EU) or FCC/UL recognition (US), whereas food and pharma companies might need approvals such as EU Organic, USFDA or WHO-GMP.

Industry experts from the pharma sector highlighted the importance of global certification stating that, “In today's globalised and highly competitive marketplace, customers will not necessarily take time to check each point individually. They will demand certifications from internationally accredited bodies. For example, EcoVadis provides Environment, Health and Safety (EHS) certification. Governments such as the US FDA, K FDA, EDQM, PMDA and Indian FDA check plants frequently and give compliance certificates or cite deviations if they notice them.”
To stay aligned with global frameworks, BIS ensures many Indian standards mirror ISO norms. The National Accreditation Board for Testing and Calibration Laboratories (NABL), with over 8,509 accredited labs, ensures test results are accepted worldwide through international recognition agreements.

Smooth clearance at ports often comes down to getting documentation right. Mr. Sailesh Shah highlighted that “Smooth clearance in most markets depends on three critical documents: a properly coded commercial invoice, a certificate of origin or EIC-issued compliance certificate and a detailed packing list supported by lab reports or health certificates. Equally critical is packaging and labelling. Destination-specific requirements, from allergen declarations in the EU to batch coding in the US, must be built into labels from the outset. Seeking pre-approval for labels from accredited inspection bodies saves both time and money.”
Mr. Sidharth Agrawal reinforced this point, noting, “Commercial Invoice: accurate product details, value, and HS codes; Bill of Lading (B/L): required to claim goods at the destination port and Packing List: helps customs verify package contents.”
Labelling and packaging are also central to export success. As Mr. Gautam Raghuraman emphasised, “Labelling and packaging are a very crucial part of exports. Any miss in this will outrightly lead to rejection of the shipment. Exporters must check with the importer and buyer for a detailed list of required markings, symbols, and language specifications. Take sample labels from them and get their approval before proceeding further. They are well-versed with local regulations, knowledge, and experience. There are also consultants available for any targeted country where exporters can opt to work with them at an additional cost.”
Building buyer confidence through consistency
Consistency in quality underpins buyer trust. India’s certification regimes ensure products are repeatedly made to standard, reducing variability. Exporters who use BIS-certified inputs and undergo EIC inspections can demonstrate “tested and verified” performance to overseas customers.
According to Mr. Sailesh Shah, “Holding certifications is only part of the equation; exporters must also deliver on key quality Key Performance Indicators (KPI). Low defect rates, on-time delivery and minimal rejection rates are benchmarks that reassure global buyers.” Mr. Sidharth Agrawal added that “An EIC certificate shows that the product has already been inspected and approved by a recognised Indian authority before shipment.

For new exporters, this helps by reducing the chances of inspection holds at the destination port, speeding up customs clearance and building buyer and customs trust, especially when exporting food, engineering goods, or regulated items.”
The benefits are clear. Strengthening organic certification alone is projected to help India cross Rs. 8,810 crore (US$ 1 billion) in organic exports by 2025-26. The number of export shipments carrying accepted quality certificates has doubled in a decade, from 61,000 to over 1,20,000.
For Micro, Small & Medium Enterprises (MSME), budget constraints often force tough decisions. Mr. Sailesh Shah recommended, “For MSMEs facing budget constraints, a smart sequence is vital. Mandatory certifications should always come first, followed by market-access certifications such as FDA, GFSI and finally, value-add certifications such as Organic or Fairtrade that enable premium positioning.”
Mr. Gautam Raghuraman stressed the importance of operational KPIs, explaining that “Being very clear with the production to delivery timeline needs to be communicated well in advance so it will help the buyer plan their shipment in advance. Additional measures need to be taken regarding packing so there is minimum impact on any kind of product rejection or defect.”
Certifications as branding and market access tools
Quality marks and labels also serve as branding tools. Products bearing credible certifications can command premium prices and easier entry. For example, India’s Halal certification (now unified under the QCI’s i-CAS Halal scheme) reassures buyers in the Middle East and other markets about religious compliance.
Sustainability labels are becoming significant as well. In October 2024, India revamped its Eco-mark Scheme, setting stringent environmental criteria for products ranging from cosmetics to electronics. Eco-labelled products are the signs of responsible production, and appeal to the eco-sensitive customers around the globe.

Digital tools can further strengthen buyer confidence. Mr. Gautam Raghuraman noted, “QR codes are an easier way to get the certificate validated or exporters can also have all the certificates mentioned/displayed on their website.” Similarly, Mr. Sailesh Shah pointed out that “Digital tools such as QR codes on packaging or links to certificate repositories allow buyers to instantly verify compliance. This transparency can be a powerful marketing tool, particularly when leveraged alongside ISI, BIS, or other authentic marks in promotional material.”
An industry expert from the pharma sector added, “Applying for international certifications (Quality, ESG) helps us assess our internal processes and standards, improvise to meet enhanced global requirements, and improve overall efficiencies. It also showcases capabilities to overseas customers, giving a competitive edge and standardising operations. In a nutshell, international certifications help maintain business continuity and attract global customers without tedious scrutiny. Every organisation seeking growth must work on both internal (safety, compliance, sustainability/environmental aspects) and external (regulatory, quality) dimensions.”
Infrastructure and future trends
India has been building on its quality infrastructure. Its ISO-17025-accredited laboratories grew to 78 in 2024-25 as compared to the previous 21 in 2013-14 and are currently capable of providing faster testing of samples and decreasing delays. Authorities are also simplifying processes through digitalisation. EIC’s planned end-to-end portal, which integrates traceability, online reporting, Internet of Things (IoT)-based sampling and e-signing, is set to streamline export clearance.
First-time exporters can make the most of regional labs to cut delays. Mr. Sidharth Agrawal advised, “Contact labs early to learn about their schedules, book tests in advance, double-check proper samples, keep lab authorities well-informed and if feasible make use of multiple labs to avoid jam-ups.”
India is aligning itself in the future with Environmental, social and governance (ESG) standards and global sustainability paths. Announcement of new rules by Eco-Mark in October 2024 is one such example. Additionally, initiatives such as “One District, One Product” and Vision 2030 mean certification facilities must increasingly reach every region.
Collaborative roadmap
New digital platforms are linking exporters with government services to ease certification. For example, Trade Connect, launched in September 2024, provides real-time trade information, connects exporters with officials, and offers access to e-certification services. The Directorate General of Foreign Trade’s (DGFT) Jan-Sunwai videoconferencing facility also allows exporters to interface directly with officials across the country, reducing delays and improving transparency.
India is also pursuing bilateral Mutual Recognition Agreements (MRAs) so that Indian quality certifications are accepted abroad. EIC has already signed protocols with China, Russia, Thailand, Bhutan, and the European Union. BIS also engages in bilateral cooperation with foreign standards bodies. Such agreements mean Indian inspection certificates are often sufficient for import clearance, reducing duplicate checks.
Standards organisations are working closely with academia and industry too. BIS has signed Memorandum of understandings (MoU) with premier institutions such as Indian Institute of Technology (IIT) and Indian Institute of Science (IISc) to integrate standardisation into research and curriculum. Accreditation bodies such as National Accreditation Board for Testing and Calibration Laboratories (NABL) and National Academy of Clinical Biochemistry (NABCB) under QCI also engage with global counterparts, strengthening India’s position internationally.
Customs cooperation is advancing through Authorised Economic Operator (AEO) programmes. An AEO-certified Indian exporter enjoys expedited clearance in partner countries such as the USA, UAE, Australia, and South Korea. India is also negotiating further agreements with Japan, Singapore, the UK, and the EU to expand these benefits.
Path forward
Looking ahead, India aims to fully leverage quality certification as a competitive edge. Officials have set a target of US$ 2 trillion in total exports by 2030. Achieving this vision will require continued digitalisation, scaled-up infrastructure in underserved areas and closer alignment with global norms. Outreach and training programmes for MSMEs will also be key in building awareness and capabilities.
By systematically strengthening its certification ecosystem through labs, standards, MRAs and digital platforms, India is laying the groundwork for making its products synonymous with quality. With these measures in place, exporters in India can earn buyer trust worldwide and contribute meaningfully to the nation’s Vision 2030 export target.
Certification Tips
Disclaimer: This information has been collected through secondary research. The views expressed by the spokespersons are their own and do not necessarily reflect those of IBEF. IBEF is not responsible for any errors in the same.




