Advantage India
Skilled human
capital
*With a total population of 1.4 billion, 47% being under the age of 25, India has a large pool of young and skilled workforce.
*India has a large reservoir of scientific human resources including scientists and engineers.
*Biotechnology has emerged as a trending career option among the youth. According to a survey of Class 12th students in Delhi, Biotechnology was ranked as the preferred stream at No.4/5.
Infrastructure
facilities
* India has 12 DBT-supported biotechnology parks and 95 BIRAC-supported bio-incubators (75 Bio-NEST and 20 E-Yuva centres), as of August 2025.
* Minister of State (IC) of the Ministry of S&T, Dr. Jitendra Singh inaugurates the Northeast's first International Biotech Conclave.
*In February 2023, Serum Institute of India announces centre of excellence in Hyderabad to tackle future pandemics.
* In July 2023, 15 companies sign MoUs worth Rs. 2,000 crore (US$ 239.99 million), in a day, for investments in biotech sector.
Policy
Support
* In August 2024, the Indian government introduced the BioE3 Policy (Biotechnology for Economy, Environment, and Employment) to promote high-performance biomanufacturing, aiming to drive green growth by enhancing R&D, fostering innovation, and creating jobs in sectors.
*The Department of Biotechnology (DBT), Government of India, is driving a transformative shift in North East India (NER) by integrating biotechnology with biodiversity conservation and economic growth. Since 2010, DBT has allocated 10% of its annual budget to specialized programs in NER, fostering research, education, and entrepreneurship. Biofoundries and biotech clusters represent a crucial step in India’s journey towards becoming a global leader in biotech innovation. With continued government support and industry collaboration, the sector is poised to break new ground, empowering startups to turn their ideas into impactful ventures.
Epidemiological
factors
* Patient pool expected to increase over 20% in the next 10 years, mainly due to rise in population.
* New diseases & lifestyle changes to boost demand for drugs and devices.
BIOTECHNOLOGY CLUSTERS
- Haryana
- Bengaluru
- West Bengal
- Maharashtra

Industry Contacts
- Department of Biotechnology, Ministry of Science & Technology
- Department of Science and Technology, Ministry of Science and Technology
- Biotechnology Industry Research Assistance Council (BIRAC)
- Council of Scientific and Industrial Research (CSIR)
- Association of Biotechnology Led Enterprises (ABLE)
- The Biotech Research Society, India
Posters
MORE
CATALYSING GROWTH
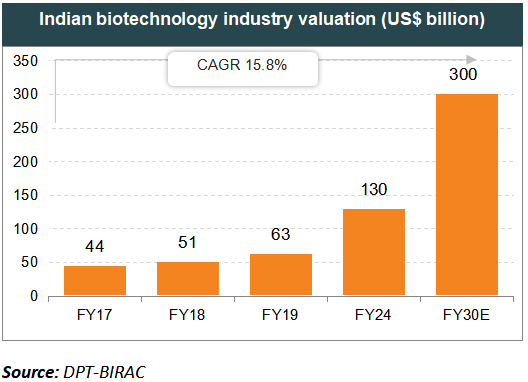
The Indian biotechnology industry amounted to US$ 63 billion in 2019 and is forecast to reach US$ 150 billion by 2025.
IBEF Campaigns
MORE
Aatmanirbhar Bharat Utsav 2024
Union Minister of External Affairs, Dr. S. Jaishankar and Union Commerce an...
Case Studies
MOREIBEF BLOG
MOREHow the Chemical Industry is preparing for a Sustainable Future
In India, the chemical industry remains one of the most influential sectors...
India’s Music Industry Today: Streaming High, Growing Fast
The Music industry in India is witnessing unprecedented growth, fuelled by ...
The Impact of Research & Development on Biotech Companies in India
India’s biotechnology sector has been on a remarkable growth trajecto...